1.38 describe the formation of a covalent bond by the sharing of a pair of
electrons between two atoms
A covalent bond is a bond formed between atoms by sharing electrons (one each) with other atoms. This leaves them stable, as they now have a full outer shell.
For example, Hydrogen has just one electron in their first and only shell, and need one more to become stable. Chlorine has seven electrons in it's outer shell and therefore needs only one more electron, so the two atoms form a bond in which they share an electron, which gives hydrogen and chlorine the extra electron it needs for a full outer shell. Once bonded in this manner, hydrogen and chlorine form hydrogen chloride (HCl).
1.39 understand covalent bonding as a strong attraction between the bonding
pair of electrons and the nuclei of the atoms involved in the bond
There is a strong attraction between the electrons being shared by atoms in a covalent bond and the nuclei of all atoms involved in the bond. This is because the nucleus has a positive charge, (composed of protons and neutrons) and the electrons have a negative charge.
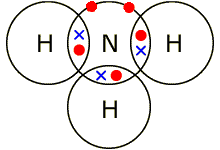
1.40 explain, using dot and cross diagrams, the formation of covalent compounds
by electron sharing for the following substances; i) hydrogen, ii) chlorine, iii) hydrogen chloride, iv) water, v) methane, vi) ammonia, vii) oxygen, viii) nitrogen, ix) carbon dioxide, x) ethane, xi) ethene.
A dot and cross diagram is constructed by drawing the outer shells of the atoms that are bonding, with an overlap in which the electrons they share should lie. All of the electrons on one of the shells should be dots, and crosses on the other so it is clear to see which electrons belong to each atom.
i) Hydrogen (H2)
Hydrogen atoms have just one electron, and need one more to become stable. Two hydrogen atoms can form a covalent bond to become stable. They are then known as hydrogen, or H2.
ii) Chlorine (Cl2)
Chlorine atoms each have seven electrons in their outer shell. Because of this, they each need one more electron to have a full outer shell and become stable. Two chlorine atoms can react to become stable and form Cl2.
iii) Hydrogen chloride (HCl)
As hydrogen and chlorine both need only one electron to have a full outer shell, they can react and become stable. This forms hydrogen chloride, or HCl.
iv) Water (H2O)
Oxygen has six electrons in it's outer shell and needs two more to become full. An oxygen atom can react with two hydrogen atoms (both need one more electron) to become stable. It has now become water (H2O).
v) Methane (CH4)
Carbon has four outer electrons, so needs four more to become full. Hydrogen atoms each have one electron, so four of these atoms bonding with one carbon atom will give carbon a full outer shell of 8 and hydrogen full outer shells with 2 electrons each. This is now called methane (CH4.)
vi) Ammonia (NH3)
Nitrogen has five outer electrons, and needs three more to become stable. It forms a bond with three hydrogen atoms, which each have one electron, to become stable. This is now known as ammonia (NH3).
vii) Oxygen (O2)
In oxygen gas, one oxygen atom shares two pairs of electrons with another oxygen atom to form a double covalent bond. They each have six electrons in their outer shell and react with eachother, sharing two electrons each and becoming stable.
viii) Nitrogen (N2)
Nitrogen atoms need three more electrons to become stable, so two nitrogen atoms can share three pair of electrons to fill their outer shells and create a triple bond.
ix) Carbon dioxide
Carbon atoms have four electrons in their outer shell and need another four to become full. Oxygen atoms each have six electrons in their outer shell and each share two of these with carbon, becoming stable. This has now formed two double covalent bonds and has become carbon dioxide (CO2).
x) Ethane (C2H6)
In ethane, six hydrogen atoms each share their only electron with one of the two carbon atoms. These two carbon atoms in return share their last electrons with eachother in a single covalent bond. This produces Ethane (C2H6).
xi) Ethene
Here, four hydrogen atoms share their only electron with one of the two carbon atoms. In return, the two carbon atoms share their last two electrons with eachother to form Ethene, a carbon-carbon double bond.
1.41 understand that substances with simple molecular structures are gases or
liquids, or solids with low melting points
The atoms within a molecule are held together by very strong covalent bonds, but the forces of attraction between these molecules are very weak. Because of the weak forces between the molecules, the melting and boiling points are very low as the molecules are easily parted from eachother. Therefore, most substances with simple molecular structures are gases, liquids or solids with low melting points.
1.42 explain why substances with simple molecular structures have low melting
and boiling points in terms of the relatively weak forces between the
molecules
A substance with a simple molecular structure is one that only contains a few atoms in a molecule.The forces between these molecules are weak, and because of this it does not take much energy or heat to be supplied in order to break them up. They therefore have low melting and boiling points as not much heat or energy is needed to be supplied to break them up.
1.43 explain the high melting and boiling points of substances with giant covalent
structures in terms of the breaking of many strong covalent bonds
A substance with a giant covalent substance will have many atoms bonded together. The forces between these molecules will be very strong as there are so many of the bonds, and that means it will take a lot of energy or heat to break them up. Because of this, substances with giant covalent structures have very high melting and boiling points.
1.44 draw diagrams representing the positions of the atoms in diamond
and graphite
Structure of diamond. Each carbon atom forms four covalent bonds in a very rigid giant covalent structure. It's because of this structure that diamond is the hardest natural substance in the world.
Structure of graphite. Each carbon atom forms three covalent bonds, creating layers which are free to slide over eachother. This leaves free electrons, which make for a good conductor of electricity, so graphite is the only non-metal which is a good conductor of electricity.
1.45 explain how the uses of diamond and graphite depend on their
structures, limited to graphite as a lubricant and diamond in cutting.
Diamond is the hardest natural substance in the world as it has so many bonds held together in such a rigid giant covalent substance. Because of this, it is extremely hard and is therefore great for cutting as it could cut anything.
In graphite, each carbon atom forms three covalent bonds which creates layers which are free to slide over eachother as the forces between them are so weak. This means that it is a slippery substance and can be used as a lubricant.













We make the custom synthesis process more efficient and cost effective while maintaining the highest standards of quality and reliability. 1-pentyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide
ReplyDeleteThe online slot machine infographic on the online casino has a 100% extra casino and everyone is tolerable to visit the online slot robot website. The best casino slot, now casino is a great top online slot website in Malaysia. The most lovely and fascinating mobile slot games are uniquely augmented than casino slots. https://918kiss2020.com/tag/infographic/
ReplyDelete